Details of the Drug
General Information of Drug (ID: DMWX683)
| Drug Name |
Mupirocin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bactoderm; Bactroban; Centany; MRC; Mupirocina; Mupirocine; Mupirocinum; Plasimine; Bactroban Nasal; Mupirocina [Spanish]; Mupirocine [French]; Mupirocinum [Latin]; Pseudomonic acid; Pseudomonic acid A; BRL 4910A; BRL-4910A; Bactroban (TN); Centany (TN); Mupirocin (USP/INN); Mupirocin, 14C-Labeled; 5,9-Anhydro-2,3,4,8-tetradeoxy-8-[[3-(2-hydroxy-1-methylpropyl)oxiranyl]methyl]-3-methyl-[2E,8[2S,3S(1S,2S); 8-Carboxyoctyl (E)-4-(2S,3R,4R,5S)-5-((2S,3S,4S,5S)-2,3-epoxy-5-hydroxy-4-methylhexyl)-3,4-dihydroxytetrahydro-2H-pyran-2-yl)-3-methylcrotonat; 9-[(E)-4-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[[(2S,3S)-3-[(2S,3S)-3-hydroxybutan-2-yl]oxiran-2-yl]methyl]oxan-2-yl]-3-methylbut-2-enoyl]oxynonanoic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteriaStaphylococcus aureusStreptococcus pyogenes
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
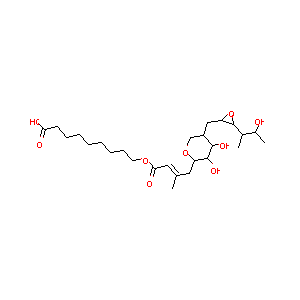 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 500.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 17 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
